Research
Biosensors
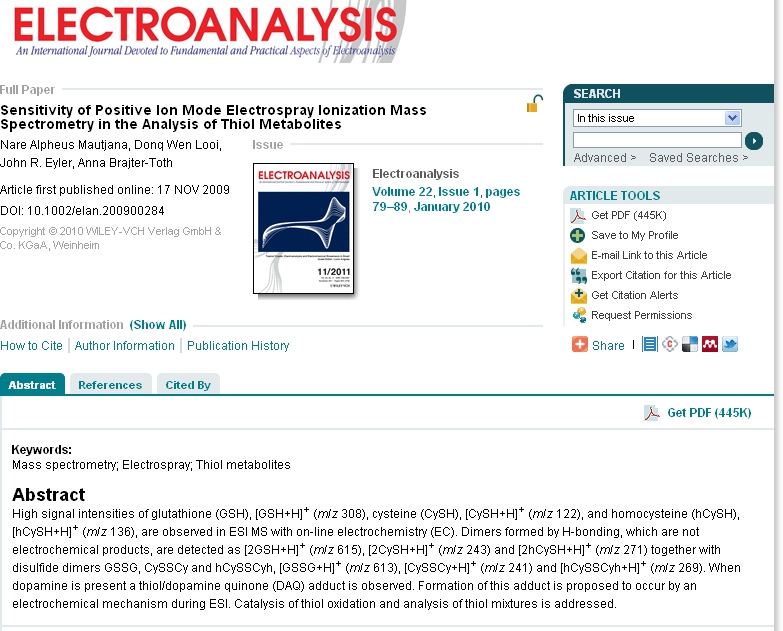
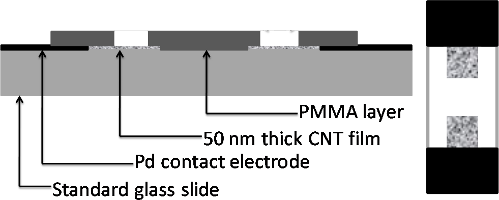
Carbon fiber microdisk electrode
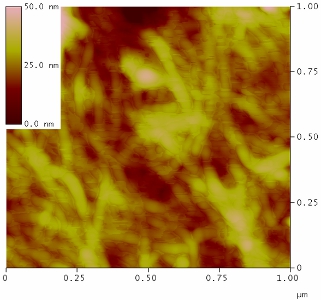
AFM image of a polyacrylonitrile (PAN) carbon fiber surface
Biosensors have wide applications both in everyday lives such as pregancy tests and blood glucose monitoring and in the laboratory. In fact, breakthroughs in electrochemical (EC) detection have miniturized glucose meters which provide fast, accurate results and reduced testing discomfort to diabetic patients. In Toth lab, we continue to push boundaries of EC biosensor technology in sensitivity, detection and range of applications to biologically relevant molecules. Like glucose, the molecules such as the signaling molecules adenosine and 2,8-dihydroxyadenine provide important clues to physiological conditions. Fabrication and sensitivity improvements of biosensors remain the primary goals of this lab. The biosensors are coupled with detection techniques such as fast scan cyclic voltammetry and FT ac voltammetry. The paper below illustrates recent progress:
Electrochemistry at Nanomaterials
SEM image of carbon nanotube surface
Schematic of a carbon nanotube electrode
Ultramicroelectrodes (< 10 μm dia.) from graphitic nanomaterials are excellent electrochemical biosensing platforms for achieving submicromolar limits of detection (LOD). Our research interest is in the development of new strategies for achieving nanomolar LOD. We have investigated optimization of sensitivity of the electrochemical biosensors with focus on the effects of graphitic nanomaterial properties. We are also interested in developing highly permeable nanoporous, ultrathin membranes that adhere to the nanostructured sensor surface and offer potential for nM LOD. Current investigations are into new applications of the nanostructured surfaces to electrochemical immunoassays, in the field of quorum sensing, and ultimately in real complex biological samples. The paper below illustrates recent progress:
Electrochemistry Electrospray Ionization Mass Spectrometry
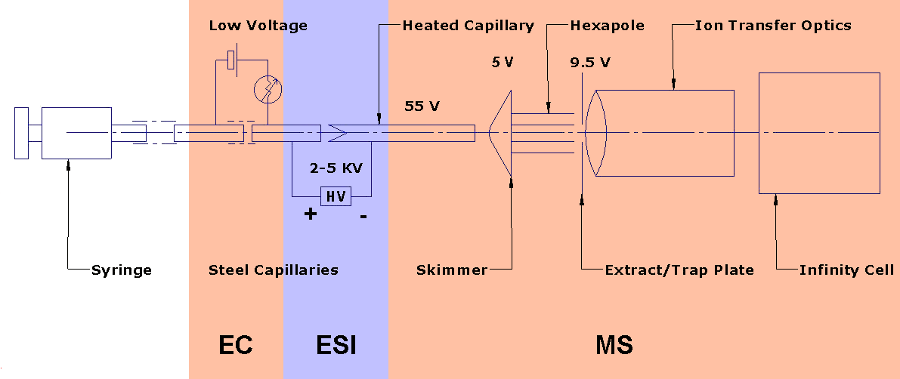
Schematic of the EC ESI MS system
Electrochemistry coupled to electrospray ionization mass spectrometry (EC ESI MS) takes advantage of the inherent electrochemical nature of ESI and combines it with a floated, voltage controllable EC cell. The ESI needle is used as an electrode in the online EC cell. A 4.7 T Bruker Daltonics Fourier Transform Ion Cyclotron Resonance mass spectrometer is used as the detector, which provides high mass resolution and high mass accuracy.
In the investigations of oxidation processes of biologically relevant molecules no additional oxidizing agents are required to generate the transient and final oxidation products. The electrochemical reactions can be studied in situ since the oxidation and reduction occurs as a result of the applied ESI voltage that can be fine tuned by the voltage applied to the EC cell. The aim is to develope the new technique for the study of new reactions such as radical oxidation pathways and product formation. Examples of applications of this technique are illustrated below:
DNA-protein crosslinks
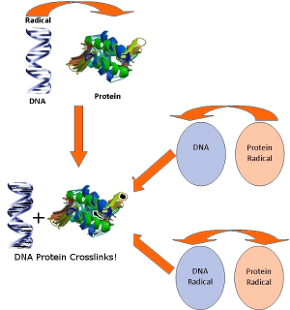
Radical initiated formation of DNA-protein crosslinks
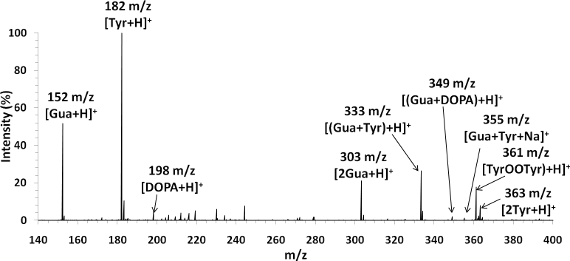 Mass spectrum of guanine-tyrosine (50µM) mixture in 50/49/1 vol% MeOH/H2O/HAc. Flow rate 50µL/hr. Guanine and tyrosine are nucleobase and amino acid respectively and act as DNA, protein analogues.
Mass spectrum of guanine-tyrosine (50µM) mixture in 50/49/1 vol% MeOH/H2O/HAc. Flow rate 50µL/hr. Guanine and tyrosine are nucleobase and amino acid respectively and act as DNA, protein analogues.
The paper below illustrates recent progress:
Electrochemistry of Metabolites
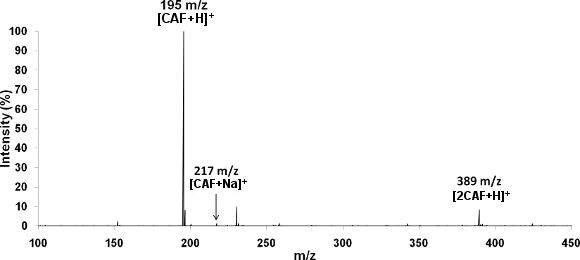
Mass spectrum of caffeine (50µM) in 50/49/1 vol% MeOH/H2O/HAc, flow rate 50µL/hr
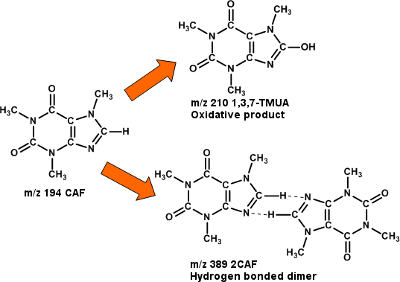
Proposed caffeine products formed in EC ESI MS
The electrochemistry of metabolites is studied as part of the wider field of metabolomics. EC ESI MS offers a fast and quick method to profile electrochemical products of known metabolites such as caffeine (CAF) and tryptophan. Identifying and characterizing these products provides information about oxidative stress at biological cells and possible markers they might leave behind. The paper below shows recent progress: